规格:95%
外观:淡黄色至棕色固体
用途:基础原料、有机中间体、医药中间体
合成工艺如下:
methanol;
Reactants are commercially availlable.
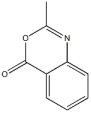
Easy and efficient strategy was undertaken to synthesize the target quinazoline Schiff?s base which involves the following three steps. In the first synthesis step, a warm solution of methyl anthranil ate reacts with acetic anhydride in 20mL methanol, results in the formation of a cyclic compound 2-methyl-4H-benzo [d] [1,3] oxazin-4-one. The second step involves the reaction between 2-methyl-4H-ben zo [d] [1,3] oxazin-4-one and hydrazine hydrate in 20mL hot methanol to afford 3-amino-2-methylquinazoline-4(3H)-one [18]. Finally, in the third step the simple condensation reaction between 3-amino-2 -methyl quinazoline-4-one and 2-hydroxy-1-naphthaldehyde results in the formation of the Schiff?s base (E)-3-((2-hydroxynaphthalen-1-yl) methyleneamino)-2-methylquinazoline-4(3H)-one HNMAMQ as present ed in supplementary file Fig. S1. The progress of the reaction was continuously monitored by the aid of thin layer chromatography (TLC) on a silica gel-G coated aluminum plates (Merck) and spots were visualized by exposing the dry plates to iodine vapors.
Feel free to call us on
025-85998075
Drop us a line anytime at
sales@popebiotech.com,
and we’ll get back
soon.
Come visit us at 2 Qiande Road, Life Science High Tech Zone, Jiang Ning District, Nanjing, Jiangsu Province, China