酮的对甲基磺酰腙在有机锂试剂作用下经烯基锂中间体生成烯烃。与Bamford-Stevens反应类似,是从酮合成烯烃的方法。Shapiro反应也是制备烯基锂中间体的方法,后者可与亲电试剂反应,从而在有机合成获得应用。
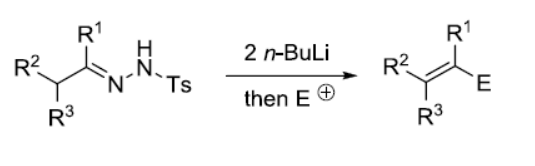
反应机理
与Bamford-Stevens反应相比,Shapiro反应的中间体为双负离子,而非卡宾,因而发生重排的倾向小。但产物的E/Z选择性一般不高。
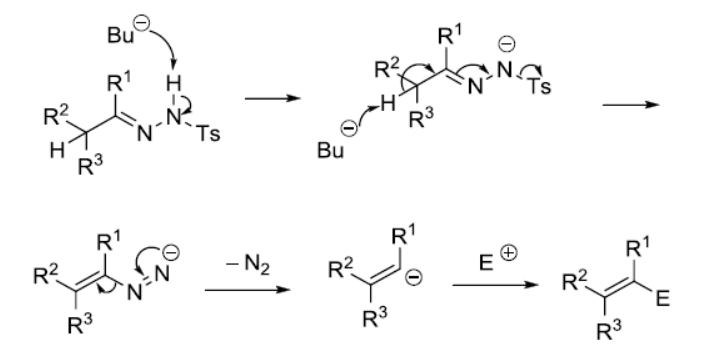
反应实例
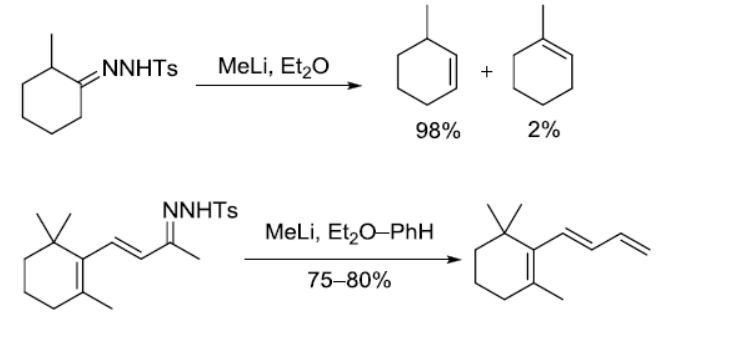
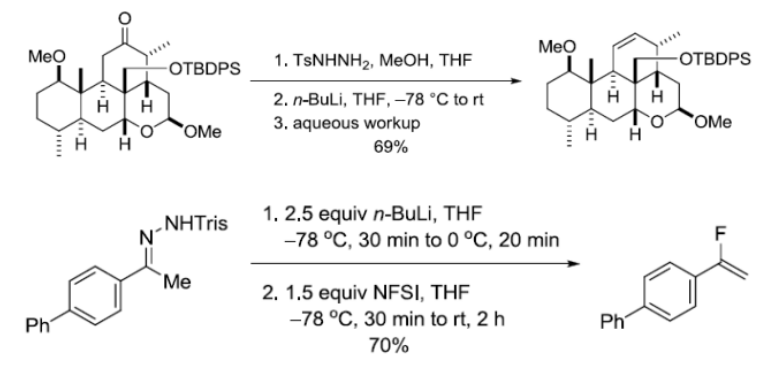
参考文献
1. Shapiro, R. H.; Duncan, J. H.; Clopton, J. C. J. Am. Chem. Soc.1967, 89, 471–472.
2. Shapiro, R. H.; Heath, M. J. J. Am. Chem. Soc. 1967, 89, 5734–5735.
3. Dauben, W. G.; Lorber, M. E.; Vietmeyer, N. D.; Shapiro, R. H.;Duncan, J. H.;Tomer, K. J. Am. Chem. Soc. 1968, 90, 47624763.
4. Wolfe, J. P. Shapiro reaction. In Name Reactions for FunctionalGroup Transformations; Li, J. J., Corey, E. J., eds, Wiley: Hoboken, NJ, 2007,pp 405-413.
5. Bettinger, H. F.; Mondal, R.; Toenshoff, C. Org. Biomol. Chem. 2008, 6, 3000–3004.
6. Yang, M.-H.; Matikonda, S. S.; Altman, R. A. Org. Lett. 2013, 15, 3894–3897.
Feel free to call us on
025-85998075
Drop us a line anytime at
sales@popebiotech.com,
and we’ll get back
soon.
Come visit us at 2 Qiande Road, Life Science High Tech Zone, Jiang Ning District, Nanjing, Jiangsu Province, China