Bischler-Mohlau吲哚合成反应亦称Bischler吲哚合成反应。从α-卤代芳香酮和过量的苯胺环化生成3-芳基吲哚。
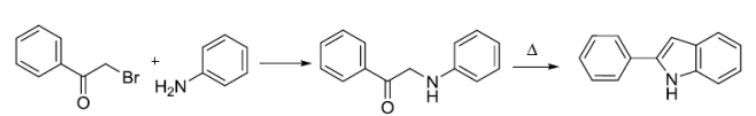
反应机理
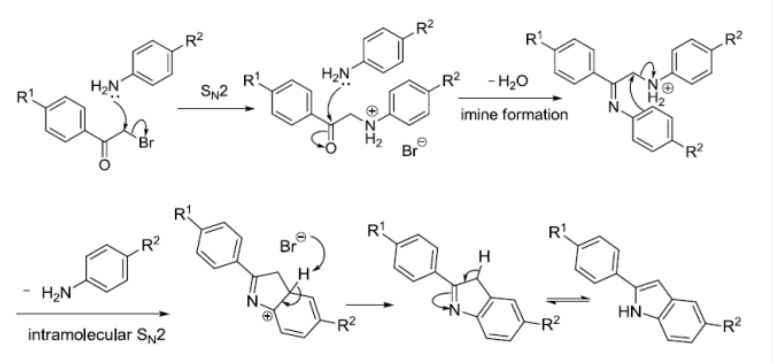
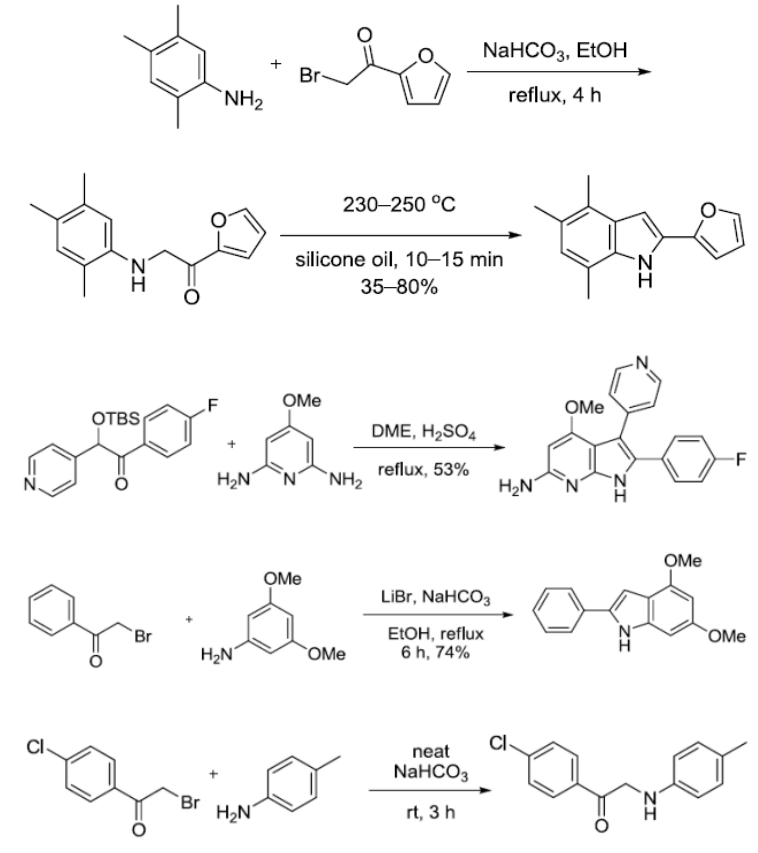
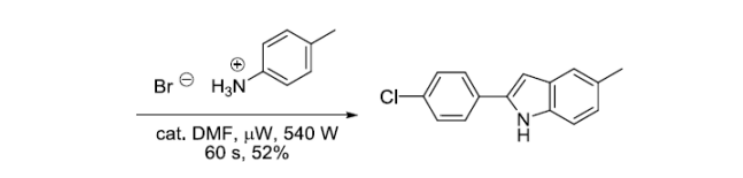
反应实例
参考文献
1. Möhlau, R. Ber. 1881,14, 171–175.
2. Bischler, A.; Fireman, P. Ber.1893, 26, 1346–1349. Augustus Bischler (1865-1957)
3. Sundberg, R. J. TheChemistry of Indoles; Academic Press: New York, 1970, pp 164.
4. Buu-Hoï, N. P.; Saint-Ruf,G.; Deschamps, D.; Bigot, P. J. Chem. Soc. (C) 1971,
2606–2609.
5. Houlihan, W. J., Ed.; TheChemistry of Heterocyclic Compounds, Indoles (Part 1),
Wiley: New York, 1972.(Book).
6. Bigot, P.; Saint-Ruf, G.;Buu-Hoï, N. P. J. Chem. Soc., Perkin 1 1972, 2573–2576.
7. Bancroft, K. C. C.; Ward, T.J. J. Chem. Soc., Perkin 1 1974, 1852–1858.
8. Coïc, J. P.; Saint-Ruf, G.;Brown, K. J. Heterocycl. Chem. 1978, 15, 1367–1371.
9. Henry, J. R.; Dodd, J. H. TetrahedronLett. 1998, 39, 8763–8764.
10. Pchalek, K.; Jones, A. W.;Wekking, M. M. T.; Black, D. S. C. Tetrahedron 2005, 61,
77–82.
11. Sridharan, V.; Perumal, S.;Avendaño, C.; Menéndez, J. C. Synlett 2006, 91–95.
12. Zhang, J. Bischler–MöhlauIndole Synthesis, In Name Reactions in Heterocyclic
Chemistry II; Li, J. J., Ed.; Wiley: Hoboken, NJ, 2011, pp 84-90.(Review).
Feel free to call us on
025-85998075
Drop us a line anytime at
sales@popebiotech.com,
and we’ll get back
soon.
Come visit us at 南京市江宁区科学园乾德路5号