烷基硅烷用过氧酸被立体选择性地氧化为相应的烷基醇。
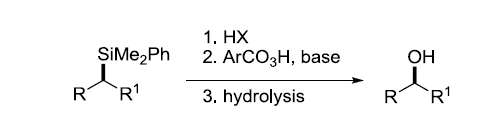
反应机理
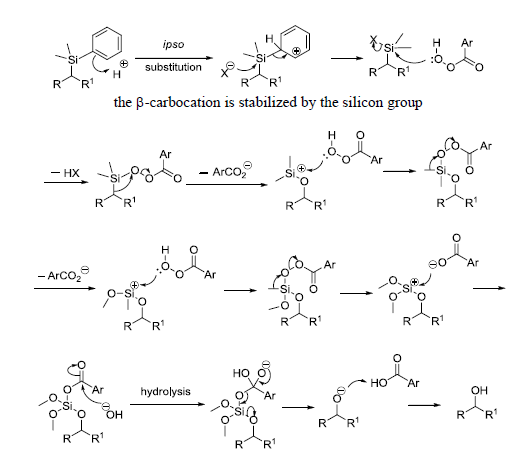
反应实例
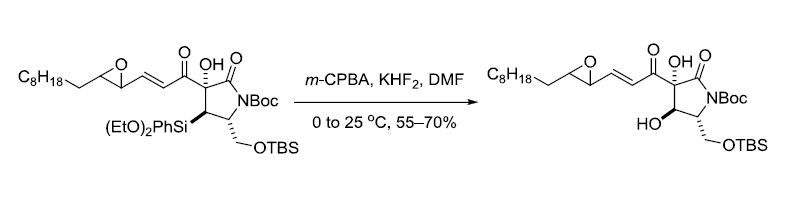
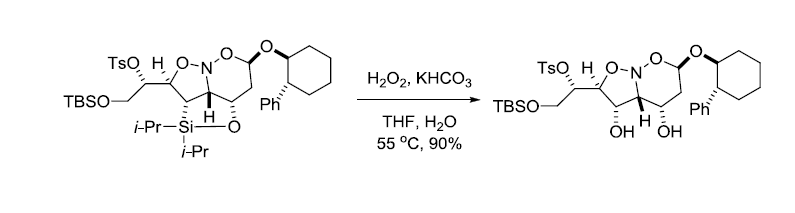
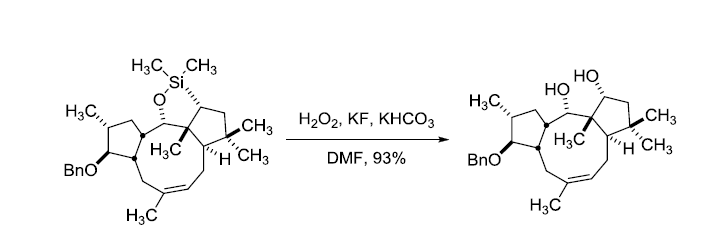
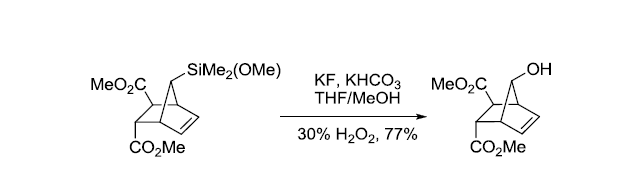
参考文献
1. (a) Fleming, I.; Henning,R.; Plaut, H. J. Chem. Soc., Chem. Commun. 1984, 29-31.
(b) Fleming, I.; Sanderson, P.E. J. Tetrahedron Lett. 1987, 28, 42294232. (c)Fleming,
I.; Dunoguès, J.; Smithers, R. Org.React. 1989, 37, 57-576. (Review).
2. Hunt, J. A.; Roush, W. R. J.Org. Chem. 1997, 62, 1112-1124.
3. Knölker, H.-J.; Jones, P.G.; Wanzl, G. Synlett 1997, 613-616.
4. Barrett, A. G. M.; Head, J.;Smith, M. L.; Stock, N. S.; White, A. J. P.; Williams, D. J.
J. Org. Chem. 1999, 64, 6005-6018.
5. Denmark, S.; Cottell, J. J.Org. Chem. 2001, 66, 4276-4284.
6. Lee, T. W.; Corey, E. J. Org.Lett. 2001, 3, 3337-3339.
7. Jung, M. E.; Piizzi, G. J.Org. Chem. 2003, 68, 2572-2582.
8. Paquette, L. A.; Yang, J.;Long, Y. O. J. Am. Chem. Soc. 2003, 125, 1567-1574.
9. Clive, D. L. J.; Cheng, H.;Gangopadhyay, P.; Huang, X.; Prabhudas, B. Tetrahedron
2004, 60, 4205-4221.
10. Mullins, R. J.; Jolley, S.L.; Knapp, A. R. Tamao–Kumada–Fleming Oxidation. In
Name Reactions forFunctional Group Transformations; Li, J. J.,Ed.; Wiley: Hoboken,
NJ, 2007, pp237-247. (Review).
Feel free to call us on
025-85998075
Drop us a line anytime at
sales@popebiotech.com,
and we’ll get back
soon.
Come visit us at 南京市江宁区科学园乾德路5号